End your neck and back pain. Schedule an appointment today!
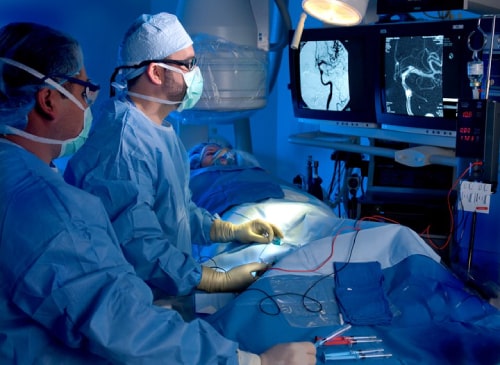
In 2004 Dr. Rappard established his medical practice in Los Angeles. At the time, the only neurointerventional/neuroendovascular surgery medical specialists in Los Angeles were located at the major medical centers of UCLA, USC and Cedars Sinai. The people of Los Angeles County having neurovascular emergencies and requiring specialized consultation were required to travel to these three facilities. In many cases, critically ill patients would suffer because hospital beds were not available to facilitate transfers to these facilities. This improved when Dr. Rappard opened his practice in the San Fernando Valley. Dr. Rappard started performing complex neurointerventional/neuroendovascular procedures at Glendale Adventist Medical Center in 2004. Soon, the facility became one of the busiest neurovascular hospitals in Los Angeles County. Dr. Rappard and his team were receiving, on average, a critically ill patient from somewhere else in the county every day of the year.

When Dr. Rappard established the first community based neuroendovascular surgery practice in Los Angeles County it meant that patients no longer had to wait in line at the big 3 facilities prior to having life saving surgery. However, many of these patients were very ill and required a specialized form of brain care called neurocritical care. Neurocritical care is intensive care designed to protect the brain from further injury. Soon, Dr. Rappard and his team became among the leading experts in neurocritical care in Los Angeles. Dr. Rappard’s neuro-ICU became so well known that care was expanded to conditions like head injury and spinal injury. Ultimately, Dr. Rappard established neurocritical care protocols at 3 community hospitals, resulting in a significant increase the neurocritical care capacity available in Los Angeles.
In 2004 Dr. Rappard opened his practice in the San Fernando Valley and made Glendale Adventist Medical Center (GAMC) his base of operations for performing endovascular operations of the brain. Common among such operations were specialized surgeries to treat clogged and ruptured brain arteries. These procedures are meant to treat or prevent stroke. Over time, the American Heart Association and The Joint Commission developed criteria for hospitals to be recognized as centers of excellence in stroke care. Working with other GAMC doctors, Dr. Rappard was instrumental in the hospital becoming the first community center in Los Angeles to achieve primary stroke center certification when it was certified in 2008. Once certification was achieved, local fire and ambulance teams began to divert critical stroke patients to GAMC for Dr. Rappard and his colleagues to perform emergency stroke care.
Intracranial atherosclerotic disease (ICAD) is an important cause of stroke. In ICAD, the small arteries of the brain become narrowed and partially blocked, restricting blood flow to the brain. The partially blocked arteries may also suddenly clot shut. Brain stenting is a surgical procedure where a small wire mesh tube, as small as 2.5 mm in diameter, is placed across a narrowed brain artery to keep it open and maintain blood flow to the brain. In 2006 Dr. Rappard was selected to become the first physician in California, and one of the first 10 in the nation, to implant a new FDA certified brain stent, the Wingspan stent, into the clogged brain arteries of a patient in a ground-breaking procedure. The procedure took place at Dr. Rappard’s community based neuroendovascular surgery practice.
One of the ways that Neuroendovascular Surgeons like Dr. Rappard treat stroke patients is by curing brain aneurysms before they rupture. A brain aneurysm is a blister in a brain artery that can pop, resulting in brain bleeding. In 2008, a glue-like substance was developed to treat special types of brain aneurysms that were notoriously difficult to treat without larger surgeries. Surgeons could navigate a micro-catheter into a brain aneurysm and fill it with glue, plugging it up before it could rupture. Rappard was selected to become the first Neurendovascular Surgeon west of the Rockies to implant the glue, called Onyx HD-500, into a patient’s brain aneurysm.
In January 2010 a near term pregnant woman in Los Angeles suffered a brain hemorrhage from a ruptured brain aneurysm. Brain aneurysms are blisters that occur in brain arteries. The blisters can pop, causing brain bleeding. The condition is fatal in about half of people. Unfortunately, the young woman had a very unusual aneurysm. It was small, with a wide base, and was arising from a small but very important brain artery. Typically, such an aneurysm would require opening the skull and performing open brain surgery to place a clip across the aneurysm base. This was fraught with risk for a near term pregnant woman. Fortunately, a brand-new balloon device had just been designed and approved for the treatment of aneurysms coming from very small brain arteries. Successful use of the balloon meant that the pregnant woman would be able to have her aneurysm plugged from the inside, without opening her brain. The operation was a success, and a young baby girl was born several days later. Rappard's emergency use of the brain balloon was the first worldwide use of the new device.
On May 31, 2011 Dr. Rappard enrolled the first patient in the United States into a trial studying the use of a patient’s own stem cells to enhance stroke recovery. The procedure involved removing bone marrow from the patient’s hip and processing the marrow in a laboratory to isolate a population of cells rich in stem cells. A catheter is then placed into a brain artery and about a teaspoon of the concentrated stem cells are injected. Study patients were compared to patients that received a simulated procedure. The trial was monitored by the FDA.














On June 20, 2010 Dr. Rappard reported that the first three patients in Los Angeles were enrolled in a trial studying the effectiveness of a device called an interspinous spacer. An interspinous spacer is a device that is inserted between two vertebra keeping the nerve passageways of the spine from narrowing and pinching nerves. The device is used to treat spinal stenosis, a condition where people suffer moderate pain and disability due to pinching of nerves in the spine. Dr. Rappard’s patients were part of a larger, nationwide cohort of patients in a study that was monitored by the FDA. The initial study results were first reported on by Dr. Rappard during the 2010 annual scientific meeting of the Society of Neurointerventional Surgery. The study resulted in multiple publications and the device was ultimately approved by the FDA.
Spinal stenosis is a condition where the nerve passageways of the spine become narrow, compressing nerves and causing pain. Until recently, there were no minimally invasive surgical options to treat patients with severe symptoms due to severe spinal stenosis. In 2014 a novel spine endoscope was developed by the Richard Wolf corporation. An endoscope is a small tube with a channel for instruments that has a lens on one end and a camera on the other. The endoscope allows the surgeon to view the inside of the spine through a small incision, allowing surgery from the inside of the spine, rather than approaching the spine from the outside. Shortly after the endoscope was developed and approved by the FDA, Dr. Rappard began to use it to treat severe spinal stenosis. Dr. Rappard has since become an authority on endoscopic surgery of the spine for spinal stenosis and regularly lectures other physicians on the subject.
In 2013 Sony introduced a heads-up display for endoscopic medical procedures. A heads-up display is a helmet worn by a physician that displays video images of surgery onto two tiny screens in front of the physician’s eyes. In 2016 Dr. Rappard became the first endoscopic spine surgeon in the United States to use the heads-up display during spine surgery. To this day, Dr. Rappard uses the device on nearly every endoscopic spine surgery procedure. The device reduces surgeon fatigue by allowing the surgeon to take a more comfortable posture during surgery. The device also increases awareness and accuracy by displaying high resolution images millimeters from the surgeon’s eyeballs, instead of making the surgeon look at a monitor across the room. The device is also 3D capable.

In 2019 Dr. Rappard’s aspirations to build Los Angeles’ most comprehensive and modern out-patient spine surgical facility was realized when he opened the Los Angeles Minimally Invasive Surgery Center. The surgical facility is home to pain management doctors, neurosurgeons, orthopedic surgeons and anesthesiologists, as well as to Dr. Rappard. In addition to its outstanding medical staff, the facility houses a unique combination of minimally invasive surgical equipment not seen anywhere else in Southern California. Modern surgical microscopes co-exist alongside state-of-the-art surgical endoscopes. The facility possesses a complete array of spine microsurgical equipment, as well as one of the largest collections of endoscopic instruments in the world. The facility is also equipped to perform common orthopedic operations. As a result, the Los Angeles Minimally Invasive Surgery Center can perform complex microsurgical procedures like disc replacements and fusions alongside complex endoscopic spine surgical procedures, a capability not seen elsewhere in Los Angeles. The facility is in Beverly Hills.

Press release on Dr. Rappard's induction.
Download PDF
Becker's Spine Review writes about
Dr. Rappard's
induction.
End your neck and back pain. Schedule an appointment today!
Contact us now to schedule your consultation or call us at (424) 777-7463 (424-77-SPINE)